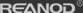Distillation VS Rectification
What is Distillation?
Distillation is a unit operation that can be used to separate homogeneous liquid mixtures. It utilises the different volatility of the components of the mixture to be separated. Volatility refers to the tendency of a substance to pass from the liquid phase into the gas phase. Examples of volatile liquids include acetone, alcohol and petrol. Distallation Plant Details
What is Rectification?
Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to fractionate crude oil. If the distillate obtained during distillation is distilled again, a new distillate is obtained with an even higher concentration of volatile components. As the procedure is repeated, the concentration of volatile components in the distillate increases on each occasion.
In practice, this multi-stage distillation process is carried out in the form of countercurrent distillation (rectification) in a column.
Key Differences between Distillation and Rectification
● Purpose
The primary purpose of distillation is to separate components based on their boiling points, whicle rectificatio aims to enhance the purity of a substance through repeated distillation cycles.
● Process Variation
While both distillation and rectification involve vaporization and condensation, rectification emphasizes repeated cycles of this process to achieve higher purity levels.
● Purity Levels
Distillation results in relatively lower purity compared to rectification. Rectification achieves higher purity levels through its multiple distillation steps.
Now we will explain distillation and reactification seperately in design technology and principle&process.
Distillation Design Technology
By using the difference in the volatility of the components in the liquid
mixture, the liquid mixture is partially vaporized and the vapor is partially
condensed, so as to achieve the separation of the components it contains. It is
a unit operation that belongs to mass transfer separation. Widely used in oil
refining, chemical, light industry and other fields.

Distillation Technology
The principle is based on the separation of a two-component mixture. The
material liquid is heated to make it partially vaporized, and the volatile
components are concentrated in the vapor, and the non-volatile components are
also concentrated in the remaining liquid, which achieves the separation of the
two components to a certain extent. The greater the difference in volatility of
the two components, the greater the degree of enrichment described above. In
industrial distillation equipment, a partially vaporized liquid phase is brought
into direct contact with a partially condensed gas phase for vapor-liquid
interstitial mass transfer. As a result, the non-volatile components in the gas
phase are partially transferred to the liquid phase, and The volatile components
are partially transferred to the gas phase, that is, partial vaporization of the
liquid phase and partial condensation of the vapor phase are achieved at the
same time.
Distillation Principle and Operation Process
Simple distillation is a batch operation process. The feed liquid is added to
a distillation kettle, and the liquid is heated and boiled under constant
pressure to continuously vaporize the liquid. The steam generated one after the
other is cold-leached and used as the top product, where the volatile components
are relatively enriched. During the distillation process, the volatile matter
concentration of the liquid in the kettle continuously decreased, and the
volatile matter concentration in the steam also decreased accordingly.
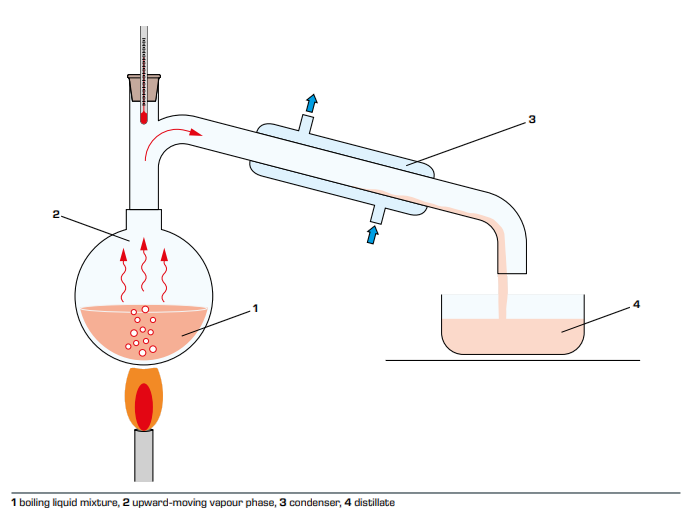
To achieve high-purity separation, rectification can be used. Distillation is
the most commonly used distillation method. One part is returned to the top of
the column, which is called reflux liquid, and the rest is continuously
discharged as the top product (distillate). In the upper part of the tower
(above the feeding position), the countercurrent contact and the material and
energy transfer are performed between the rising steam and the reflux liquid.
The bottom of the column is equipped with a reboiler (distillation kettle) to
heat the liquid to produce steam. The steam rises along the tower, contacts the
falling liquid countercurrently and conducts material energy transfer, and the
bottom of the tower continuously discharges part of the liquid as the bottom
product. The rising steam is partially condensed many times, and the temperature
gradually decreases. The concentration of volatile component A gradually
increases. The falling liquid is partially gasified many times. The temperature
gradually increases, and the concentration of non-volatile component B gradually
increases. The concentration gradually decreases, the temperature distribution
in the tower gradually decreases from the bottom to the top, and the
concentration of the A component gradually increases from the bottom to the top.
In the rectification section, the lower part of the column has completed the
concentration of the heavy components in the descending liquid, that is, the
light component is proposed, so it is called the stripping section.
In such a
column, a two-component mixture can be continuously separated High purity light
and heavy two components. It can be seen that the difference between
rectification and distillation lies in 'reflux'. Reflux is a necessary condition
for gas and liquid contact mass transfer.
Rectification Principle and Operation Process
The liquid mixture to be separated (feed) is fed to the column and partially evaporates on its way to the bottom of the column where it is heated to boiling. The vapour produced moves upwards inside the column, exits it at the top and is condensed. Part of the condensate is carried away as top product. The remainder flows back into the column and moves downwards as liquid phase.
Due to column internals, such as bubble cap trays or random packings, the downward-moving liquid phase is subjected to an intensive exchange of heat and material with the upward-moving vapour phase. The less volatile components of the vapour phase condense and increase in concentration in the liquid phase. At the same time, the condensation heat released evaporates the more volatile components of the liquid phase. These processes in the column increase the vapour phase concentration of volatile components moving from the bottom to the top of the column. The liquid phase concentration of less volatile components increases in the opposite direction, from the top of the column to the bottom.
What is the Difference Between Distillation and Rectification?Types of Distillation and Rectification
Understanding the different types of distillation helps clarify their usage scenarios:
Simple Distillation
Used when the boiling points of components differ significantly (e.g., >25°C). No trays or packing are needed.
Fractional Distillation
Used when boiling points are close. Requires a column filled with trays or packing to enhance separation.
Vacuum Distillation
Conducted under reduced pressure to lower boiling points. Ideal for thermally sensitive compounds.
Azeotropic and Extractive Distillation
Used to break azeotropes by adding solvents or third components.
Reactive Distillation
Combines chemical reactions and distillation in the same unit.
Rectification (Continuous Fractionation)
Involves multiple stages of vapor-liquid equilibrium using reflux, producing highly pure top and bottom products.
Equipment and Column Design
In both distillation and rectification, equipment configuration plays a critical role:
Tray Columns
Use sieve trays, valve trays, or bubble cap trays to increase contact between rising vapor and descending liquid.
Packed Columns
Use random or structured packings (e.g., Raschig rings, Pall rings) for high surface area and energy efficiency.
Reboiler
Located at the bottom to vaporize the liquid feed using external heat.
Condenser
Located at the top to condense rising vapor into liquid.
Reflux Drum
Stores condensed liquid. A portion is sent as product, the rest returned as reflux to improve purity.
Operation Modes: Batch vs. Continuous
Batch Distillation
Operates in cycles
Suitable for lab-scale or flexible production
Less efficient for large volumes
Continuous Distillation (Rectification)
Feed and product streams operate non-stop
Ideal for petrochemical, beverage, and pharmaceutical industries
Consistent product quality with energy optimization
Thermodynamic Principles Behind Separation
Raoult's Law
The partial vapor pressure of a component in a mixture is proportional to its mole fraction and vapor pressure.
Dalton’s Law
The total pressure of a gas mixture equals the sum of the partial pressures of each component.
Relative Volatility (α)
The ratio of vapor pressures of two components determines how easily they can be separated. The higher the α, the simpler the separation.
Energy Considerations in Distillation and Rectification
Distillation is energy-intensive and accounts for up to 40–60% of energy usage in some chemical plants.
Energy Losses: Occur due to inefficient heat transfer or poor insulation.
Heat Integration: Using multi-effect distillation, heat exchangers, or thermal coupling improves efficiency.
Advanced Control Systems: Automation and real-time sensors optimize reboiler and reflux ratios.
Environmental Impact
VOC Emissions
Volatile organic compounds released during distillation need to be captured to avoid pollution.
Waste Heat
Untreated waste heat can contribute to thermal pollution. Proper heat recovery systems are essential.
Wastewater and Residues
Bottom products may require further treatment, especially in pharmaceutical and chemical applications.
Sustainability
Energy-efficient designs and closed-loop systems are increasingly adopted to align with green chemistry principles.
Safety and Regulatory Compliance
Distillation and rectification processes involve high temperatures, flammable substances, and pressurized equipment.
Key Hazards:
Overheating or overpressure
Fire and explosion risks (especially in alcohol or solvent distillation)
Corrosive or toxic chemicals
Compliance Standards:
OSHA (Occupational Safety and Health Administration)
EPA (Environmental Protection Agency) regulations on emissions
ATEX/IECEx certifications for hazardous environments
GMP compliance for pharmaceutical and food-grade applications
Industrial Applications
| Industry | Use Case |
|---|
| Petroleum | Fractionating crude oil into LPG, gasoline, diesel, etc. |
| Pharmaceuticals | Purifying solvents and active pharmaceutical ingredients (APIs) |
| Food & Beverage | Alcohol distillation, essential oil extraction, flavor production |
| Chemical | Producing high-purity acetone, benzene, toluene, xylene |
| Environmental | Wastewater treatment through separation of pollutants |
| Semiconductor | Ultra-pure water and solvent purification |
Conclusion: Distillation vs Rectification—Which One to Choose?
Distillation is ideal for basic separation tasks, especially when component volatility differs significantly. However, for high-purity, high-efficiency, and large-scale operations, rectification is the superior choice. Its use of reflux and multiple contact stages makes it indispensable in modern chemical engineering.
Companies investing in continuous production lines or high-value chemical processing will benefit most from rectification systems. Meanwhile, distillation remains cost-effective and practical for small-batch or initial separation tasks.
SL Tec is a professional Distillation Plants contractor and distillation formula & production technology provider at low cost, we can guide you with our formular and technology to help you improve your production efficiency. Welcome to contact us to get more details of distillation production process from us at any time, and we would like to offer any technical guidance to you.