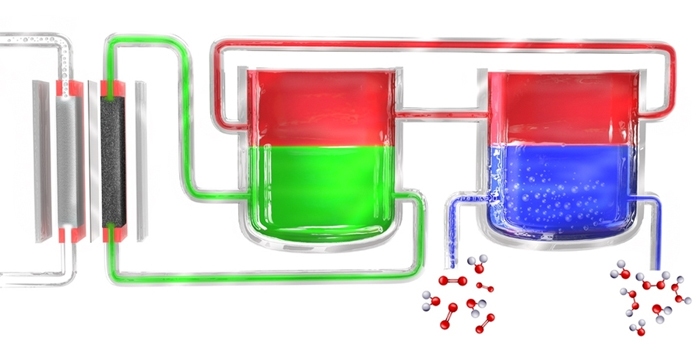
In a new method for portable production of hydrogen peroxide, an electrolyzer breaks down water into hydrogen and oxygen. Hydrogen atoms are initially formed in the electrolyte material (green), then transferred to the mediator material (red), which then takes them to a separate cell where the mediator comes into contact with oxygen-rich water (blue), where the hydrogen combines with it to form hydrogen peroxide. The mediator then returns in order to start the cycle again.
Hydrogen peroxide is a useful general purpose disinfectant that can be found in most medicine cabinets in developed countries. But in remote villages in developing countries, where it can play an important role in health and hygiene, it is difficult to obtain.
A process is now available to create a simple, inexpensive, portable device that can continuously produce hydrogen peroxide from air, water and electricity, thus providing a way to disinfect wounds, food preparation surfaces and even water supplies.
Even at low concentrations, hydrogen peroxide is an effective antimicrobial agent, and after performing its germicidal function, it breaks down into ordinary water, while other agents such as chlorine leave unwanted byproducts from its production and use.
Hydrogen peroxide is simply water plus an extra oxygen atom - it is H2O2, not H2O. The extra oxygen is relatively loosely bound, making it a highly reactive chemical that is eager to oxidize any other molecule around it. It is so reactive that it can be used as rocket fuel at high concentrations, and even concentrations of 35% require very special handling and transport procedures. The kind used in household disinfectants is usually only 3% hydrogen peroxide and 97% water.
Because high concentrations are difficult to transport and low concentrations (mainly water) are uneconomical to transport, it is often difficult to reach places where this material might be particularly useful, such as remote communities with untreated water. (Bacteria in water supplies can be effectively controlled by the addition of hydrogen peroxide.) As a result, many research groups around the world have been seeking ways to develop some form of portable hydrogen peroxide production equipment.
Most hydrogen peroxide produced in the industrialized world is produced in large chemical plants where methane or natural gas is used to provide a source of hydrogen, which is then reacted with oxygen at high temperatures in a catalytic process. This process is energy intensive, not easily scalable, and requires large equipment and a steady supply of methane, making it unsuitable for smaller units or remote areas.
There is growing community interest in portable hydrogen peroxide as it is realized that it can indeed meet many needs on the industrial side as well as human health and hygiene.
Other processes developed to date for potential portable systems have key limitations. For example, most catalysts that facilitate the formation of hydrogen peroxide from hydrogen and oxygen also produce large amounts of water, resulting in a low concentration of the desired product. In addition, processes involving electrolysis, like this new process, often have difficulty separating the hydrogen peroxide produced from the electrolyte material used in the process, again leading to inefficiencies.
Our team solved the problem by breaking the process down into two separate steps. First, electricity (preferably from solar cells or windmills) is used to break down water into hydrogen and oxygen, and then the hydrogen reacts with a "carrier" molecule. This molecule - called an anthraquinone compound in these initial experiments - is then introduced into a separate reaction chamber, where it meets oxygen extracted from the outside air and a pair of hydrogen atoms combine with an oxygen molecule (O2) to form a hydroperoxide. In this process, the carrier molecule returns to its original state and returns to re-circulate, so this material is not consumed.
This process can solve many challenges by making clean water, emergency wound care and sterile food preparation surfaces more readily available where they are currently scarce or unavailable.
Even at fairly low concentrations, you can use it to disinfect water of microbial contaminants and other pathogens. And, at higher concentrations, it can even be used to perform so-called advanced oxidation, and in combination with UV light, it can even be used to purify water from intense industrial wastes such as mining operations or hydraulic fracturing.
Thus, for example, a portable hydrogen peroxide plant could be set up near a fracking or mining site for cleaning up its effluent and then transferred to another location after the original site ceases operations.
In this initial proof-of-concept unit, the concentration of hydrogen peroxide produced is still low, but further engineering of the system should be able to produce a more concentrated output. One way to do this is to increase the concentration of the media, and fortunately we already have media at very high concentrations for the liquid flow cell, so we think there is a pathway to increase those concentrations.
It's a remarkable process because you need a lot of things, water, air and electricity, that you can source locally and then you use it to make this important chemical that you can use to really clean up the environment as well as sanitation and water quality.
The ability to make hydrogen peroxide solutions in water without electrolytes, salts, bases, etc. is noteworthy, all of which are inherent to other electrochemical processes. Obtaining salt-free aqueous solutions of H2O2 is of wide interest for practical applications.
This work represents an innovative application of 'dielectric electrolysis'. The fact that dielectric electrochemistry offers a way to combine traditional chemical processes with electrochemistry is a particularly compelling demonstration of this concept. ...... This concept has many potential applications.