The big brown bottle of hydrogen peroxide (H2O2) is a staple of the modern medicine cabinet, always on hand for first aid needs. Lesser known uses of hydrogen peroxide include disinfecting hospital equipment and fueling spacecraft. Yet as common and beneficial of a substance as it is, hydrogen peroxide is surprisingly hard to produce and transport.
Currently, hydrogen peroxide is made through what's known as the "anthraquinone process.” This method is energy-intense, requires large-scale production, and produces large quantities of carbon dioxide (CO2) as a byproduct. While directly reacting hydrogen and oxygen to make hydrogen peroxide would be ideal, thermodynamics prefers to form the more stable water (H2O) over hydrogen peroxide.
So the challenge becomes: does a material exist that can be used to selectively, reliably, and efficiently form hydrogen peroxide whenever and wherever it's needed so that transporting it isn't necessary?
With our method, we hope to provide the capability of making hydrogen peroxide when you need it, wherever you are.
The most difficult thing about hydrogen peroxide is transporting it. Hospitals and space missions have particular uses for hydrogen peroxide, including rapid sterilization and as an oxidant. Transport of hydrogen peroxide to hospitals can be dangerous and complicated, and you can't take gallons of oxidants into space due to weight restrictions, so with our method we hope to provide the capability of making hydrogen peroxide when you need it, wherever you are.
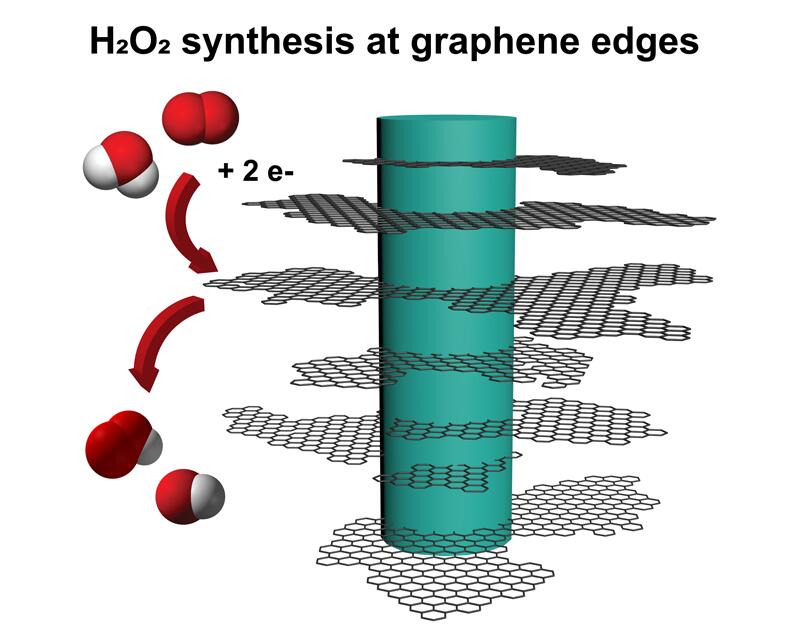
For several years, a lab has been developing a technique of growing graphene in a 3D topology, leveraging defects in the material to grow “fuzzy graphene,” which is a form of carbon, is also highly abundant, cheap, and renewable. It allows graphene to grow away from a surface, rather than along it, creating long, thin, flaky graphene structures that look somewhat like nanoscale pine trees.
Graphene has an impressive ability to transport electric charges. We found that graphene-based materials with lots of edges, like the ones found at the tips of each fuzzy graphene flake, are highly reactive for synthesizing hydrogen peroxide. So fuzzy graphene, electrically conductive material with many edges, is the perfect candidate for this new and improved method of hydrogen peroxide generation.
We can now really controllably make hydrogen peroxide with high selectivity. “We are now able to make mostly hydrogen peroxide, and not that much water. Thermodynamics really wants hydrogen and oxygen to form water when catalyzed, so being able to catalyze so selectively, with 94% of the outcome being hydrogen peroxide, means our process is highly novel.