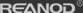It's always been asked that is acetate the same as acetone? Actually acetate vs acetone, the main difference between acetone and acetate is that acetone is a ketone, while acetate is an anion derived from acetic acid. Both acetone and acetate are studied under organic chemistry because they are organic compounds or derivatives of organic compounds. Acetone is the simplest of the ketone family, while acetate is the anion formed from acetic acid. SL Tec would give a clear explaination.
What is acetone?
Acetone is a ketone with the chemical formula (CH3)2CO. It exists as a colorless, flammable and volatile liquid at room temperature. It is the simplest and therefore the smallest ketone in the ketone family. In addition, it is the organic solvent we use in our household and industrial needs. In addition, it is a common ingredient in nail polish removers, varnishes, glues, rubber cements, and etc.
The current method of producing acetone is mainly propylene. We refer to this method as the isopropylbenzene process. It involves the reaction of benzene with propylene (alkylation of benzene) to produce isopropylbenzene, which is an organic compound based on aromatic hydrocarbons with aliphatic substituents. Through the oxidation of isopropylbenzene, we can obtain phenol and acetone. The reactions are as follows.
Application of Acetone
The main application of acetone is as a solvent. It is a good solvent for plastics and synthetic fibers. In addition, acetone is used as a raw material for the synthesis of methyl methacrylate. In addition, it can be used as a food additive.
What is Acetate?
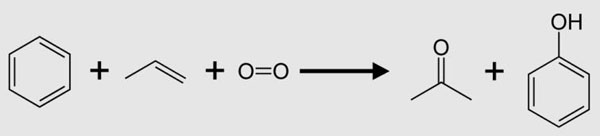
Acetate is an anion derived from acetic acid. The chemical formula of the anion is CH3COO-. We can abbreviate it as OAc. For example, we can abbreviate sodium acetate as NaOAc. If the anion combines with a hydrogen cation, acetic acid (carboxylic acid) is formed. If the acetate ion combines with an alkyl group, an ester is formed.
METHYL ACETATE PRODUCTION PROCESS FOR METHYL ACETATE PLANT
In most cases, we use the term acetate to name the acetate salts. These salts contain a combination of acetic acid with an alkali metal, an earth metal, a metal or a non-metal and another base. In addition, the term is common in biology and is the main compound used by organisms "acetyl coenzyme A".
Difference between Acetate vs Acetone
Acetates vs Acetone
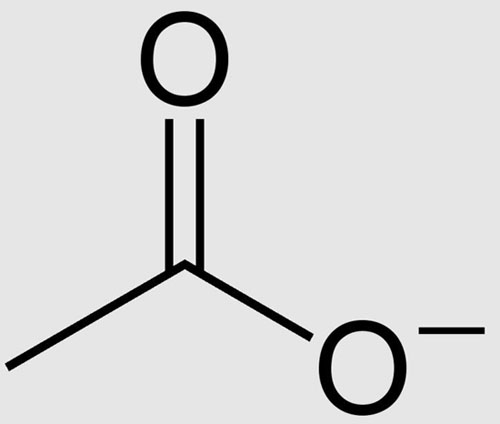
Acetone is a ketone with the chemical formula (CH3)2CO, while acetate is the anion. The main difference between acetone and acetate is that acetone is a ketone, while acetate is the anion we derive from acetic acid. Acetone is a neutral compound, while acetate has a - 1 charge.
In addition, we can produce acetone artificially through the isopropylbenzene process, but biologically it is formed in our bodies during the breakdown of fats into ketone bodies. Acetate, however, is formed by removing protons from acetic acid. The following infographic summarizes the differences between acetone and acetate in tabular form.
Acetone is an organic compound, while acetate is an anion derived from acetic acid (a carboxylic acid). The main difference between acetone and acetate is that acetone is a ketone, while acetate is an anion derived from acetic acid.
SL Tec is professional supplier of methyl acetate production process for Methyl Acetate Plant and ethyl acetate production process for Ethyl Acetate Plant. With rich experience, we would help you to increase the production amount and production efficiency of methy acetate and ethyl acetate.
Welcome to contact us to get further detailed infomation.