MEG Plant/ MEG Production
Ethylene glycol is a widely used chemical product, which is used as the raw material for solvent, antifreeze and polyester synthesis. The existing production processes for ethylene glycol include: chloroethanol method, ethylene oxide hydration method, gas-phase catalytic hydration method, direct ethylene hydration method, formaldehyde method, oxalic ester method,etc.
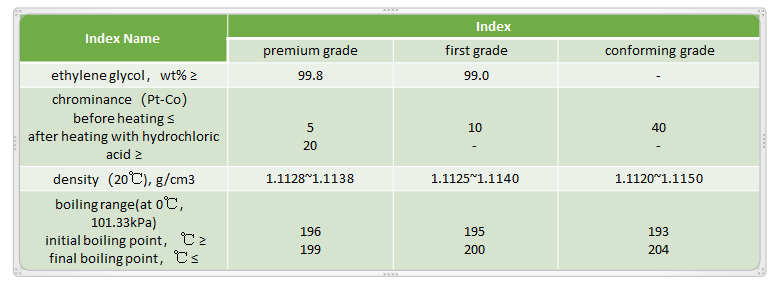
Specification of MEG Production(GB/T464)
Production Routes
There are two main routes for Ethylene Glycol (Monoethyle Glycol/MEG) production: one is the Olefin/EO(Ethylene Oxide) Route starting from either naphtha, ethane or methanol, the licensors include Shell, SD, UCC and etc. And the other is the DMO(dimethyl oxalate) Route newly emerged in China these years, starting from syngas. Depending upon the difference operation pressure, this DMO Route is further divided into Normal Pressure Process and Medium-High Pressure Process.
SL TECH offers the most advanced and the most competitive Medium-High Pressure DMO Process for MEG production. Its production cost is much lower than that of Olefin/EO Process at the current low oil price (i.e., USD 67/ BBT), not to mention the Normal Pressure DMO Route.
Welcome to contact us to get medium-high pressure DMO process for MEG production at lower cost.
Process Comparison of Oxalic Ester Methods
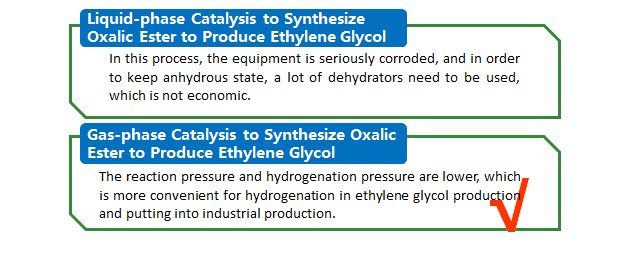
Liquid-phase Catalysis to Synthesize Oxalic Ester to Produce Ethylene Glyco
FAQ of MEG Plant
How is Meg produced through our meg plant?
Meg is produced by the hydration reaction of ethylene oxide, and then vacuum distilled to obtain pure ethylene glycol.
There are two main routes for Ethylene Glycol (Monoethyle Glycol/MEG) production: one is the Olefin/EO(Ethylene Oxide) Route starting from either naphtha, ethane or methanol, the licensors include Shell, SD, UCC and etc. And the other is the DMO(dimethyl oxalate) Route newly emerged in China these years, starting from syngas. Depending upon the difference operation pressure, this DMO Route is further divided into Normal Pressure Process and Medium-High Pressure Process.
What is Meg used for in oil and gas?
End uses for MEG range from clothing and other textiles, through packaging to kitchenware, engine coolants and antifreeze. Polyester and fleece fabrics, upholstery, carpets and pillows, as well as light and sturdy polyethylene terephthalate drink and food containers originate from ethylene glycol. The humectant (water attracting) properties of MEG products also make them ideal for use in fibres treatment, paper, adhesives, printing inks, leather and cellophane.
Related News
The Development Of MEG Plant
How Much Do You Know About MEG?
MEG Technology and Production Facilities
New MEG Production Process
MEG Agent Technology and Plants
MEG Application
Advantages & Features of MEG Plant
The MEG plant provided by SL TEC has advantages as follows:
1. The pressure of the carbonylation unit is increased to 2.0-3.0MPa, about 5-7 times the conventional process, thereby the diameter of main equipment and pipes are reduced by 2-2.5 times.
2. The carbonylation reactor is changed from tubular type to plate type, whose heat transfer effect is increased by one time, the catalyst loading coefficient increased by over 60%, the STY (Space-to-Time Yield) more than doubled, which allows the large scale of each production line.
3. The catalyst has better selectivity, higher conversion yield and longer service time ( over 2 years)
4. The CAPEX of the carbonylation unit is reduced by 50%, while the investment of the carbonylation unit takes 40% of the total investment.
Choose Slchemtech for unparalleled Monoethylene Glycol production technology and elevate your business to new heights.


MEG production process
Process overview
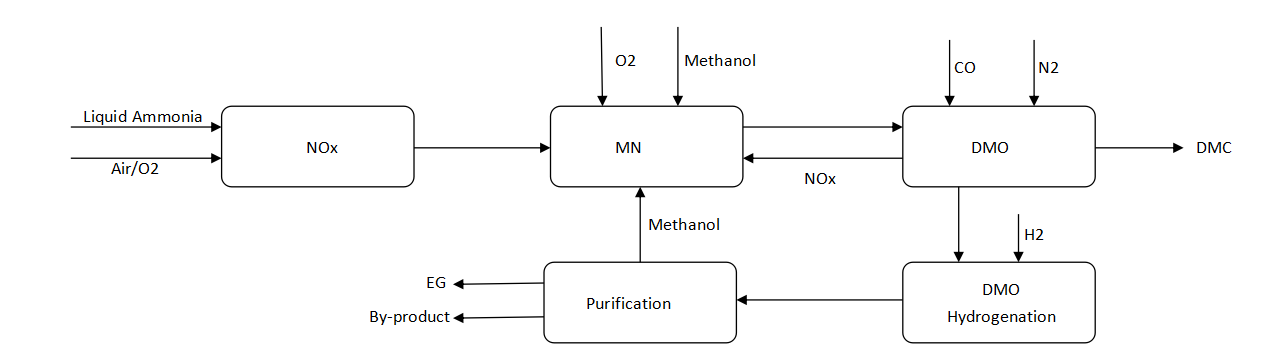
The MEG (ethylene glycol) production unit is mainly based on the DMO (dimethyl oxalate) route with synthesis gas as raw material. Liquid ammonia and air/O₂ are used as starting materials, and high-purity ethylene glycol (MEG) is finally generated through a series of complex chemical reactions and separation processes.
Process flow
NOx generation: Liquid ammonia and air/O₂ enter the NOx unit, undergo an oxidation reaction to generate NOx (nitrogen oxides), and provide key nitrogen oxide intermediates for subsequent reactions.
MN synthesis: The generated NOx reacts with the introduced O₂ and methanol in the MN unit to generate MN (methyl formate). At the same time, part of the NOx will return to the DMO unit to participate in the subsequent cycle reaction to achieve efficient utilization of materials and cycle balance.
DMO synthesis and conversion: The MN generated by the MN unit enters the DMO unit and reacts with CO and N₂ under the action of a specific catalyst to generate DMO (dimethyl oxalate). Part of DMO enters the next process as an intermediate product, and the other part undergoes hydrogenation reaction in the DMO hydrogenation unit to generate H₂. The whole process ensures high yield and high quality of DMO by precisely controlling the reaction conditions.
DMO purification and MEG generation: The crude dimethyl oxalate after DMO purification enters the EG (ethylene glycol) synthesis unit, and finally generates ethylene glycol (MEG) through multi-step reactions such as ester exchange and hydrogenation. Through advanced separation and purification technology, ethylene glycol is separated from the complex reaction mixture to obtain high-purity ethylene glycol products that meet market demand. At the same time, the separated by-products will also be effectively collected and processed to achieve comprehensive resource utilization and environmentally friendly production.


None